Stress Wrecks Your Gut’s Repair Crew: The Corticosterone Connection
Feeling Stressed? Your Gut Might Be Feeling It Too.
Okay, let’s talk about stress. We all know it, we all feel it sometimes (or maybe a lot of the time!). But have you ever stopped to think about what it’s *really* doing inside your body, beyond that knot in your stomach or that racing heart? I recently stumbled upon some fascinating research that dives deep into how psychological stress isn’t just a ‘mind thing’ – it’s got a direct line to your gut, and not in a good way.
Think of your brain and your gut as being in constant conversation. They’re linked by this incredible network called the brain-gut axis. It’s like a superhighway involving nerves, hormones, and even your immune system. When you’re stressed, this highway gets overloaded, and the messages start getting a bit… garbled. We’ve known for a while that stress can mess with things like how fast food moves through you, how much fluid is secreted, and even how ‘leaky’ your gut lining is. This is why folks under pressure often report tummy troubles, and why stress is linked to conditions like Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease (IBD).
Meet Your Gut’s Essential Repair Crew: Intestinal Stem Cells
Now, let me introduce you to some real heroes inside your gut: Intestinal Stem Cells (ISCs). These are like the ultimate repair crew. Your gut lining gets replaced constantly – it’s one of the fastest-renewing tissues in your body. ISCs are the powerhouses that divide and create all the different types of cells needed to keep that lining healthy and intact. If your ISCs aren’t happy and working properly, your gut’s ability to repair itself is compromised. And that, as you can imagine, can open the door to all sorts of problems, especially when faced with other irritants or inflammation.
Scientists have suspected that stem cells, in general, might be particularly sensitive to stress. But exactly how psychological stress specifically affects these crucial gut stem cells and what that means for your gut health hasn’t been totally clear. Sure, we knew the gut microbiome (all those helpful bacteria) plays a role in linking stress to the gut. But what about something *intrinsic* to your body, a direct signal from the brain via that brain-gut axis?
The Study’s Big Reveal: Stress Hits ISCs Directly
This new study I read about really zeroes in on that intrinsic pathway. The researchers used mouse models (a common way we study complex biological processes) to see what happens to the gut under chronic stress. They used methods like chronic restraint stress (basically, making the mice a bit uncomfortable for periods) and chronic unpredictable stress (mixing up different mild stressors). And guess what they saw? The stressed mice showed signs of depression-like behavior and, importantly, their guts were affected. Their small intestines were shorter, the crypts (where the stem cells live) were shallower, and there were fewer actively dividing cells.
But the really striking finding was about the ISCs themselves. Under chronic stress, the number of these vital stem cells went down significantly, and their ability to multiply and do their job was seriously impaired. They even tested this by taking cells from the stressed mice and trying to grow them in a dish as ‘organoids’ (mini-guts) – and the cells from stressed mice were much worse at forming and growing these organoids. This strongly suggested that stress was directly messing with the ISCs’ function.
Enter the Stress Hormone: Corticosterone Takes the Blame
So, how is the stress signal getting from the brain to the gut stem cells? The usual suspects when it comes to chronic stress are hormones released by the adrenal glands, part of the Hypothalamic-Pituitary-Adrenal (HPA) axis – another key player in that brain-gut highway. The main stress hormone in mice (and similar to cortisol in humans) is called corticosterone.
The researchers did another clever experiment: they removed the adrenal glands from some mice before stressing them. And bam! The negative effects of stress on the gut length, crypt depth, and crucially, the ISCs (their numbers and activity) were largely reversed. This pointed a big finger at something coming from the adrenal glands.
To figure out *which* adrenal signal was responsible, they gave normal mice either corticosterone or another stress-related signal (noradrenaline) via injection. Only the mice given corticosterone showed the same gut issues as the stressed mice – reduced gut length, fewer dividing cells, and fewer functional ISCs. Giving corticosterone directly to the mini-gut organoids in the dish also suppressed their growth. This was strong evidence that elevated corticosterone, caused by stress, is the key culprit directly harming the ISCs.
The Nitty-Gritty: How Corticosterone Sabotages ISCs
Science loves to know the ‘how’. So, the researchers dug into the molecular details. Corticosterone works by binding to a receptor called NR3C1 (also known as the glucocorticoid receptor) inside cells. They found that NR3C1 is present in ISCs, suggesting corticosterone could act directly on them. To test this, they genetically engineered mice where the NR3C1 receptor was specifically removed from ISCs. When these mice were stressed, their ISCs were protected! They didn’t show the same reduction in number or activity that the normal stressed mice did. This confirmed that corticosterone acts directly on ISCs via NR3C1.
Next, they looked at what genes were changing in ISCs from stressed or corticosterone-treated mice. They found changes in pathways related to cell growth and division. A key player that popped up was a gene called FKBP5, which was significantly increased by stress and corticosterone. Why is FKBP5 important? Well, it’s known to interfere with a crucial signaling pathway called AKT, which is like a ‘go’ signal for cell growth and survival. FKBP5 helps turn off AKT (specifically, it helps remove a phosphate group at a key spot, Ser473). When AKT is turned off, another protein called FOXO1 gets activated and moves into the cell’s nucleus. FOXO1 then puts the brakes on cell division by increasing proteins that stop the cell cycle.
So, the pathway looks like this: Stress -> Elevated Corticosterone -> Binds to NR3C1 in ISCs -> Increases FKBP5 -> FKBP5 inactivates AKT -> FOXO1 moves to nucleus -> Inhibits ISC proliferation. Phew! They even showed that blocking FKBP5 with a specific inhibitor (called SAFit2) could rescue the ISCs from the negative effects of corticosterone or stress. It’s a complex chain reaction, but it clearly shows how a stress hormone can directly cripple the gut’s repair machinery.
Why This Matters: Stress, ISCs, and Colitis
Okay, so stress messes with ISCs. Why should we care? Well, think about inflammatory conditions like colitis (inflammation of the colon), which is a form of IBD. The gut lining is constantly under attack in colitis, and effective repair by ISCs is absolutely critical for healing. If your repair crew is depleted and sluggish because of stress, it makes sense that your gut would struggle to heal.
The researchers tested this idea using a mouse model of colitis (induced by a chemical called DSS). As expected, chronic stress made the colitis much worse – more weight loss, more severe symptoms, shorter colons, and more damage visible under the microscope. And right alongside this aggravated inflammation, they saw a dramatic drop in ISC numbers and activity in the colon.
The crucial test was in the mice where NR3C1 was deleted specifically in ISCs. Remember, deleting NR3C1 protected the ISCs from stress. When these protected mice were stressed and then given DSS to induce colitis, they showed significantly less severe colitis compared to normal stressed mice. Their body weight was better, symptoms were reduced, colon length was less affected, and there was less damage and inflammation. While it didn’t completely prevent the stress-induced worsening of colitis (other factors like the microbiome are also involved, as the study notes), protecting the ISCs by blocking the corticosterone signal made a huge difference.
This finding is a big deal because it provides a concrete, *intrinsic* mechanism explaining why psychological stress can make gut inflammation worse. It’s not just about changes in bacteria or nerve signals; it’s about the stress hormone directly impairing the very cells needed for healing.
What This Means for You and Me
So, what’s the takeaway from all this science? For me, it really underscores how deeply connected our mental and physical health are. Your state of mind isn’t just ‘upstairs’ – it’s sending signals throughout your body, right down to the microscopic level of your gut stem cells. Chronic stress isn’t just an unpleasant feeling; it can actively undermine your body’s ability to maintain and repair one of its most vital barriers.
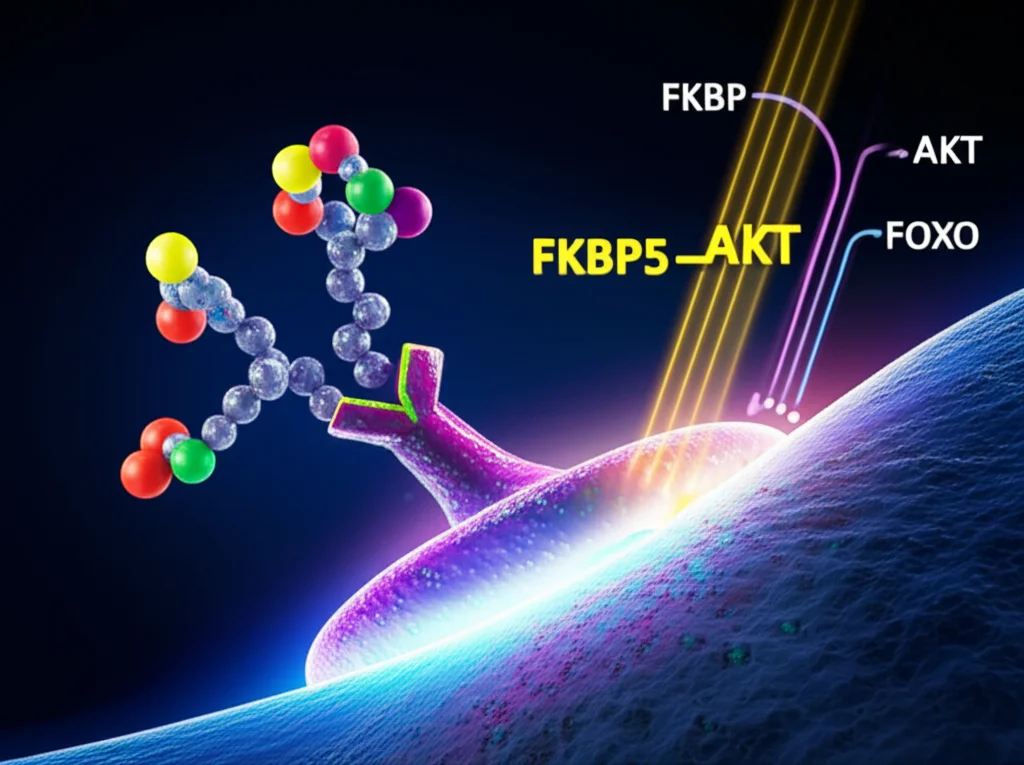
This research highlights corticosterone (and by extension, cortisol in humans) as a key mediator in this process. It suggests that finding ways to manage chronic stress isn’t just good for your mental well-being; it’s potentially crucial for protecting your gut health and reducing your susceptibility to inflammatory conditions. While this study was done in mice, the underlying mechanisms involving stress hormones and their receptors are highly conserved across mammals, making the findings highly relevant to human health.
Looking Ahead (and a Note on Mice)
Of course, science is always a journey. These studies in mice give us incredible insights, but the human gut is complex, with its own unique factors. Future research will need to confirm these exact pathways in human cells and patients. Also, the study authors note that while they saw stress affect ISC numbers and proliferation, another recent study found stress affecting ISC *differentiation* (what types of cells they turn into), possibly via the microbiome. This suggests there are multiple ways stress can impact ISCs, and they might interact. It just shows how intricate the brain-gut connection truly is!
But even with those nuances, this research provides a powerful, mechanistic explanation for something we’ve long suspected: chronic psychological stress can directly sabotage your gut’s ability to repair itself, making you more vulnerable to conditions like colitis. It’s a compelling reminder that taking care of your mind is a fundamental part of taking care of your body.
Source: Springer