Unlocking Diabetic Kidney Damage: The SNHG7, miR-181b-5p, and TLR4 Connection
Hey there! Let’s Talk Kidneys and Diabetes
So, you know how diabetes can mess with different parts of the body? Well, one of the toughest spots it hits is the kidneys. We call it Diabetic Kidney Disease, or DKD for short. It’s a really big deal globally, often leading to serious kidney failure. Honestly, even with the treatments we have now, keeping DKD from getting worse is a real challenge. That’s why finding new ways to understand and fight it is super important.
Think of your kidneys as incredible filters, working tirelessly. But in DKD, high blood sugar causes stress that can really damage these filters. One way this damage happens is through something called pyroptosis. Now, pyroptosis sounds a bit dramatic, right? It’s a specific kind of cell death, almost like a controlled explosion, but in DKD, it goes haywire in the kidney cells, especially the tubular ones. This doesn’t just kill cells; it also triggers a nasty inflammatory party, making the whole situation worse and leading to scarring and damage.
Meet the Molecular Crew: SNHG7, miR-181b-5p, and TLR4
Our cells are buzzing with activity, driven by tiny molecules. Among them are long non-coding RNAs (lncRNAs) and microRNAs (miRNAs). They don’t make proteins themselves, but they’re like conductors, telling other molecules what to do. One lncRNA we’ve been looking at is called SNHG7. And there’s a miRNA, miR-181b-5p, which previous studies hinted might be important in kidney issues and inflammation.
Turns out, SNHG7 and miR-181b-5p seem to be linked. Online tools predict that SNHG7 can actually bind to miR-181b-5p. Think of SNHG7 acting like a tiny sponge, soaking up miR-181b-5p. When miR-181b-5p is sponged up, it can’t do its usual job. And what’s one of miR-181b-5p’s jobs? Keeping a protein called TLR4 in check.
What We Found: The Plot Thickens
We got some kidney samples from people with DKD and compared them to samples from people without it. And guess what? In the DKD samples, we saw that SNHG7 and TLR4 levels were high, while miR-181b-5p levels were low. This lines up with our “sponge” idea – high SNHG7 soaking up miR-181b-5p, leading to more TLR4.
To dig deeper, we used kidney cells in the lab (called HK-2 cells) and treated them with high glucose to mimic the diabetic environment. The same thing happened! SNHG7 and TLR4 went up, and miR-181b-5p went down. It seems this molecular imbalance is a key feature in DKD kidney cells.
Putting the Brakes On: What Happens When We Interfere?
This is where it gets interesting from a potential treatment perspective. We tried reducing the amount of SNHG7 in our high-glucose lab cells. And wow, the results were pretty encouraging! Lowering SNHG7:
- Helped the cells survive better.
- Reduced cell damage (measured by something called LDH leakage).
- Calmed down the inflammatory response, lowering levels of pesky inflammatory signals like TNF-α and IL-1β.
- Suppressed that dramatic cell death process, pyroptosis, by affecting key players in that pathway (like NLRP3 and caspase-1).
On the flip side, increasing SNHG7 made everything worse, just as we’d expect if it’s driving the problem.
The Chain Reaction: SNHG7 -> miR-181b-5p -> TLR4 -> NF-κB -> Trouble!
Our experiments strongly suggest that SNHG7 acts like that sponge for miR-181b-5p. And miR-181b-5p directly targets TLR4. So, the sequence seems to be: High SNHG7 sponges miR-181b-5p, which frees up TLR4, leading to more TLR4 activity.
Why is TLR4 activity a problem here? Because TLR4 is known to activate another major signaling pathway in cells called NF-κB. And NF-κB is like a master switch for inflammation and other stress responses. When NF-κB gets activated, it tells the cell to produce more inflammatory molecules and can trigger pyroptosis.
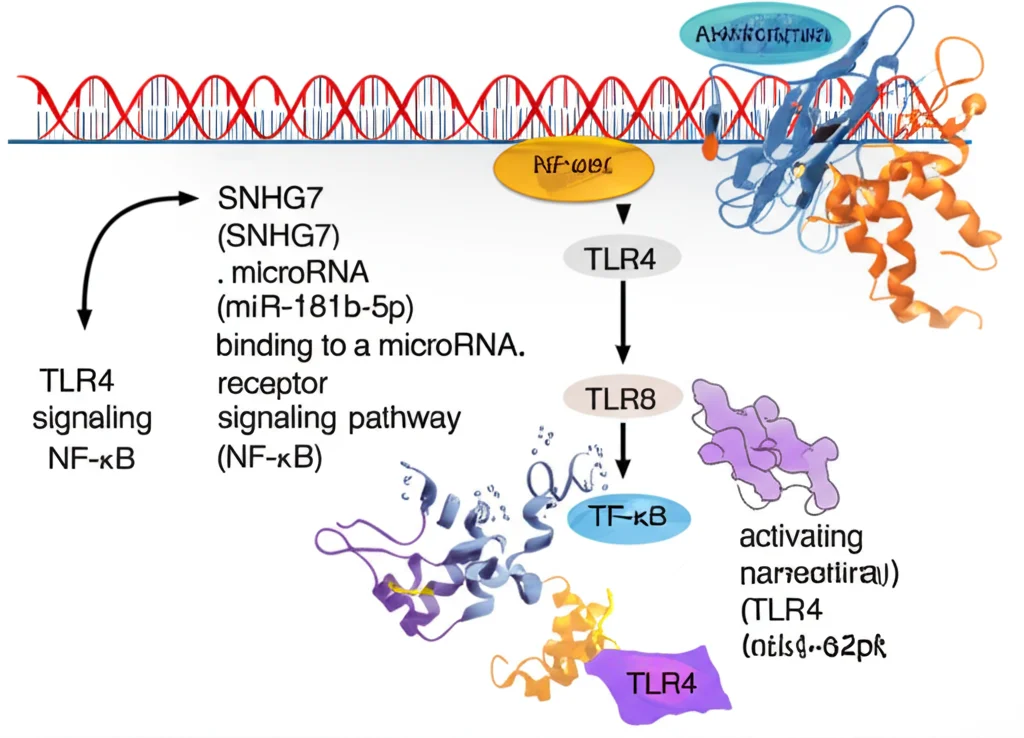
We tested this idea by blocking miR-181b-5p or boosting TLR4 even when SNHG7 was low. Doing that reversed the protective effects of lowering SNHG7. This really solidifies the idea that the SNHG7/miR-181b-5p/TLR4 axis is the critical chain of command here, ultimately leading to NF-κB activation, inflammation, and pyroptosis in the kidney cells under high glucose stress.
The Big Picture: Why This Matters
So, what’s the takeaway from all this molecular detective work? We’ve uncovered a key pathway – SNHG7 acting as a sponge for miR-181b-5p, leading to increased TLR4 and activation of the NF-κB pathway – that drives inflammation and pyroptosis in kidney cells during diabetes. This is a pretty big deal because it points to SNHG7, or perhaps other players in this specific chain, as potential targets for new therapies for DKD.
Imagine if we could develop a treatment that specifically blocks SNHG7 or boosts miR-181b-5p activity in the kidneys. Based on our lab results, this could potentially put a stop to the damaging inflammation and cell death that makes DKD so hard to manage. It’s a promising avenue for future drug development.
Looking Ahead: Still Questions to Answer
Of course, science is a journey, and we’re not at the finish line yet. Our study had some limitations. For instance, the number of patient samples was relatively small. We also need to figure out if these molecular changes *cause* the kidney damage or happen alongside it, and how they relate to different stages of DKD. And turning these findings into an actual treatment involves a lot more research, including making sure any potential therapy is safe and can get to where it needs to go in the body.
But despite these challenges, identifying this specific SNHG7/miR-181b-5p/TLR4/NF-κB pathway as a central player in DKD inflammation and pyroptosis is a significant step forward. It gives us a concrete target to aim for as we continue the search for more effective ways to protect the kidneys of people living with diabetes.
Source: Springer